Menu
- Practice Areas
Over 18 Years of Helping Clients when they need It Most
- Results
- About
- News
- Contact
Over 18 Years of Helping Clients when they need It Most
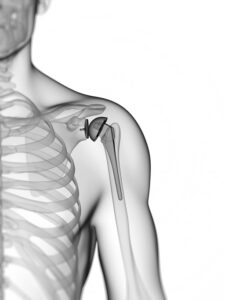 The product liability attorneys of Murray Guari Trial Attorneys are investigating alleged claims against Zimmer Biomet’s Reverse Shoulder implant.
The product liability attorneys of Murray Guari Trial Attorneys are investigating alleged claims against Zimmer Biomet’s Reverse Shoulder implant.
The Comprehensive Reverse Shoulder device, manufactured by Zimmer Biomet, is used by thousands of patients who undergo shoulder surgery for rotator cuff tear, arthropathy arthritis, or to fix previously failed shoulder joint replacements. The Comprehensive Reverse Shoulder device is surgically implanted to help restore arm movement. The device simulates the humerus bone and socket of the shoulder.
Alleged side effects and injuries from the use of the Comprehensive Reverse Shoulder device include:
In 2017, the U.S. Food & Drug Administration issued an urgent Class 1 recall, the most serious type of recall, as these devices may cause serious adverse health consequences, injuries or death. The device has a high rate of fracturing, more so than what is stated on the product’s label.
The defective products law firm of Murray Guari Trial Attorneys represents patients who have been affected by the Zimmer Biomet Comprehensive Reverse Shoulder implant, on a contingency basis which means that there are never any legal fees unless we win compensation in your case. For a free no-obligation consultation please call toll free at 1-877-645-2974.
Please note that in some matters, we may co-counsel or refer the case to another lawyer or law firm.
For a FREE consultation, simply leave your details below and we’ll contact you soon.